Avogadro's Number Is Useful For
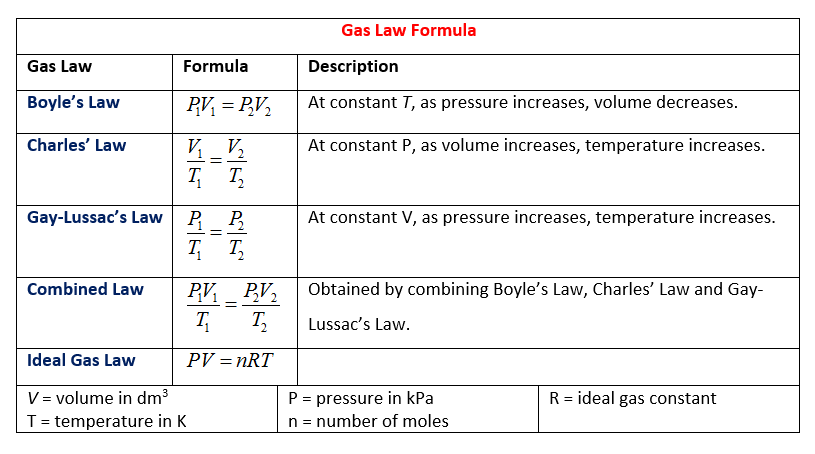
Avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. The units may be electrons, atoms, ions, or molecules, depending on the nature of the substance and the character of the reaction (if any).See alsoAvogadro’s law. 5.11x10 23 atoms of lithium is equal to how many moles? (to find moles, you must divide atoms by Avogadro's number).
Avogadro's Number Is Equal To One
12 O6
O6
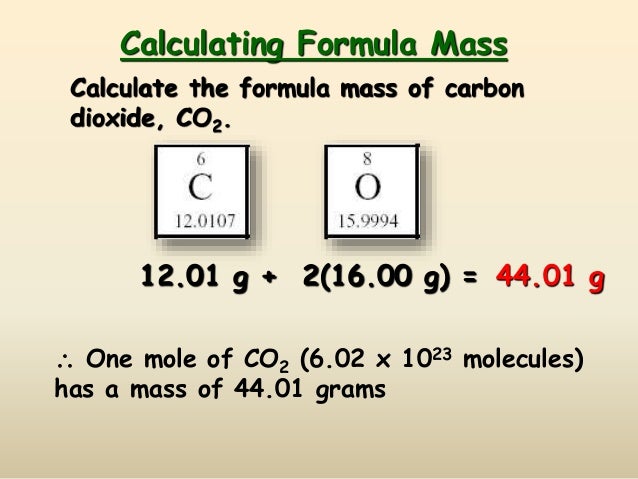
 , contains 6.02252×1023 molecules of glucose. Avogadro's number is determined by calculating the spacing of the atoms in a crystalline solid through X-ray methods and combining this data with the measured volume of one mole of the solid to obtain the number of molecules per molar volume.
, contains 6.02252×1023 molecules of glucose. Avogadro's number is determined by calculating the spacing of the atoms in a crystalline solid through X-ray methods and combining this data with the measured volume of one mole of the solid to obtain the number of molecules per molar volume. The Columbia Electronic Encyclopedia, 6th ed. Copyright © 2012, Columbia University Press. All rights reserved.
See more Encyclopedia articles on: Chemistry: General
